TIME’s annual list of THE BEST INVENTIONS features “200 extraordinary innovations changing lives.” To compile the list, TIME solicited nominations from its editors and correspondents around the world, and through an open online application process, paying special attention to growing fields, such as AI, green energy, and sustainability. TIME then evaluated each contender on a number of key factors, including originality, efficacy, ambition, and impact. (For more information, please visit http://www.time.com/best-inventions-2023)
LEQEMBI is the first and only treatment approved in Japan and the United States shown to reduce the rate of disease progression and to slow cognitive and functional decline, that acts on the underlying pathology of AD.
AD is a progressive, fatal disease, and a global healthcare issue that greatly impacts not only the people living with the disease, but also their loved ones, care partners and society. Based on our corporate concept of “human health care (hhc),” we have taken on the challenge of this difficult issue through our nearly 40 years of drug discovery activities in the field of dementia, while spending time with patients and their families. We will deliver LEQEMBI to the people with early AD who need it and their families, and aim to continue creating impact on global issues surrounding dementia.
Eisai serves as the lead of LEQEMBI development and regulatory submissions globally with both Eisai and Biogen Inc. (U.S.) co-commercializing and co-promoting the product and Eisai having final decision-making authority.
Please see full Prescribing Information, including Boxed WARNING in the United States.
MEDIA CONTACTS:
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
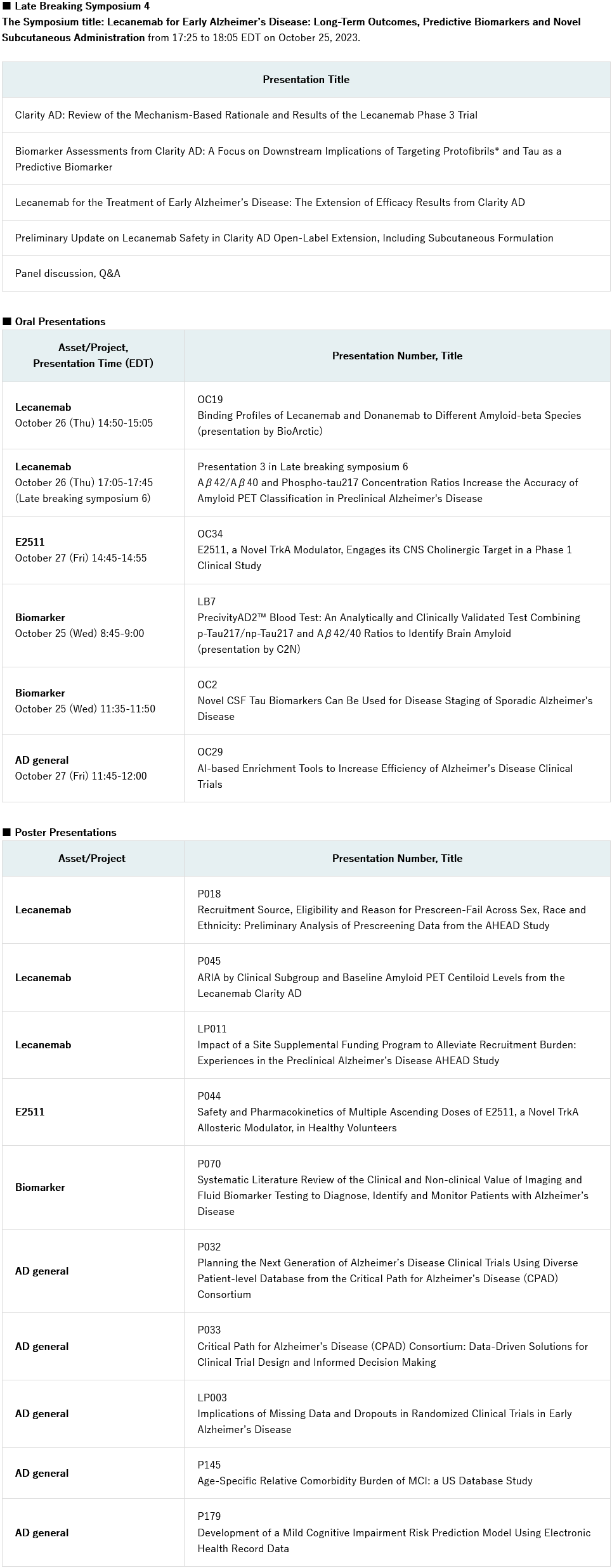
Eisai Co. Ltd (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that the company will present new data from the phase 3 Clarity AD study for its Alzheimer’s disease (AD) treatment LEQEMBI? (lecanemab-irmb) 100 mg/mL injection for intravenous use and new data on the subcutaneous formulation in development at the 16th annual Clinical Trials on Alzheimer’s Disease (CTAD) conference. The conference will be held in Boston, Massachusetts, United States and virtually from October 24 to 27, 2023. In addition to the data presented on Eisai’s anti-amyloid beta (Aβ) protofibril* antibody LEQEMBI, phase 1 data for E2511, an investigational tropomyosin receptor Kinase A (TrkA) positive allosteric modulator (PAM), will be presented as well as other research from the company’s AD pipeline. At the conference, Eisai will present data and research in five oral and ten poster presentations. BioArctic will give an oral presentation on lecanemab.
Late-Breaking Symposium 4 – Lecanemab for early Alzheimer’s Disease: Long-Term Outcomes, Predictive Biomarkers, and Novel Subcutaneous Administration
- In a late-breaking symposium on October 25 from 17:25-18:05 EDT, Eisai will present the latest data from the Clarity AD optional tau PET longitudinal substudy. The presentation will include a post-hoc analysis of the low and intermediate + high-tau subgroups, with the low-tau subgroup representing early stages of disease studied specifically in the phase 3 core study, and the open-label extension study. An update on the investigational subcutaneous formulation, including interim safety and effect on amyloid in the brain measured by amyloid PET, will be provided.
- Distinguished faculty members Christopher van Dyck M.D., Keith Johnson M.D. and Reisa Sperling M.D. will discuss the findings in a panel led by Michael Irizarry, M.D., MPH, Eisai.
- A live webcast of this symposium can be viewed on the?Eisai Co., Ltd. website.
“Alzheimer’s disease is a progressive and relentless condition that requires early diagnosis and continued treatment. LEQEMBI supports neuronal function in Alzheimer’s disease by clearing highly toxic protofibrils that can continue to cause neuronal injury and death well after plaques are cleared,” said Michael Irizarry, MD, MPH, Senior Vice President, Clinical Research, Neurology, Deputy Chief Clinical Officer, Clinical Evidence Generation, Eisai. “We look forward to sharing the new LEQEMBI low-tau subgroup data and subcutaneous data at CTAD 2023.”
Other major oral presentations include:
- Lecanemab: Binding profiles of lecanemab and donanemab to different amyloid-beta species (OC19, presentation by BioArctic).
- E2511, a novel TrkA modulator, engages its CNS cholinergic target in a phase 1 clinical study (OC34).
- Novel CSF tau biomarkers can be used for disease staging of sporadic Alzheimer’s disease (OC2).
The full list of presentations about Eisai assets and research follows.
This release discusses investigational uses of agents in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that such investigational agents will successfully complete clinical development or gain health authority approval.
MEDIA CONTACTS:
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
Eisai Inc. (U.S.)
Libby Holman
+ 1-201-753-1945
Libby_Holman@Eisai.com
Eisai Europe, Ltd.
(UK, Europe, Australia, New Zealand and Russia)
EMEA Communications Department
+44 (0) 786 601 1272
EMEA-comms@eisai.net
Notable presentations include a post-hoc analysis of tumor response by baseline characteristics of the metastases from the pivotal Phase 3 CLEAR (Study 307)/KEYNOTE-581 trial, which evaluated lenvatinib (LENVIMA?), the orally available multiple receptor tyrosine kinase inhibitor discovered by Eisai, plus pembrolizumab (KEYTRUDA?), anti-PD-1 therapy from Merck & Co., Inc., Kenilworth, versus sunitinib for the first-line treatment of patients with advanced renal cell carcinoma (NCT02811861; Presentation: #1903P). An exploratory analysis from the pivotal Phase 3 Study 309/KEYNOTE-775 trial of outcomes for patients with advanced endometrial cancer who completed treatment with pembrolizumab and continued with lenvatinib will also be presented (NCT03517449; Presentation: #748P).
“As a research and development-focused company driven by our hhc (human health care) concept, we strive to make a difference in the lives of patients and their families by advancing the science of cancer medicine with our robust portfolio and pipeline,” said Dr. Takashi Owa, Chief Scientific Officer, Senior Vice President, Eisai Co., Ltd. “At this year’s ESMO meeting, analyses from the pivotal Phase 3 CLEAR and Study 309/KEYNOTE-775 trials may provide greater insights into the treatment of patients with advanced renal cell carcinoma and certain types of advanced endometrial carcinoma. We also look forward to sharing data for lenvatinib and from our pipeline, as well as engaging in critical scientific exchange with the community in service of moving oncology research forward.”
Additional data from the LEAP (LEnvatinib And Pembrolizumab) clinical program to be presented include safety-run-in results from the Phase 3 LEAP-014 trial evaluating lenvatinib plus pembrolizumab and chemotherapy as a treatment option for patients with metastatic esophageal squamous cell carcinoma (NCT04949256; Presentation: #1534P). A network meta-analysis of lenvatinib versus key comparators as first-line treatment for patients with unresectable hepatocellular carcinoma will also be presented during a poster session (Presentation: #1007P).
Research from Eisai’s pipeline will be featured in a poster presentation of findings from the dose-expansion portion of a Phase 1 study evaluating E7389-LF, a liposomal formulation of eribulin, as a potential first-line chemotherapy treatment option for patients with metastatic/advanced HER2-negative breast cancer (Presentation: #405P). Additionally, insights from preclinical research on farletuzumab ecteribulin (FZEC, formerly known as MORAb-202), a folate receptor alpha (FRα)-targeting antibody drug conjugate (ADC), in endometrial cancer will be presented (Presentation: #786P).
This release discusses investigational compounds and investigational uses for FDA-approved products. It is not intended to convey conclusions about efficacy and safety. There is no guarantee that any investigational compounds or investigational uses of FDA-approved products will successfully complete clinical development or gain FDA approval.
The full list of presentations is included below. These abstracts will be made available via the ESMO website on Monday, October 16, 2023, at 12:05 AM CEST.

In?March 2018, Eisai and Merck & Co., Inc., Rahway, NJ, USA (known as MSD outside?the United States?and?Canada), through an affiliate, entered into a strategic collaboration for the worldwide co-development and co-commercialization of lenvatinib, both as monotherapy and in combination with the pembrolizumab, anti-PD-1 therapy from Merck & Co., Inc., Kenilworth, NJ, USA.??Eisai and Merck & Co., Inc., Kenilworth, NJ, USA are studying the lenvatinib plus pembrolizumab combination through the LEAP (LEnvatinib And Pembrolizumab) clinical program in various tumor types across multiple clinical trials.
In June 2021, Eisai and Bristol Myers Squibb entered into an exclusive global strategic collaboration agreement for the co-development and co-commercialization of FZEC. Eisai and Bristol Myers Squibb are currently investigating FZEC in multiple studies including: a Phase 1/2 clinical study for select solid tumors including endometrial cancer, a Phase 2 clinical study for non-small cell lung cancer, and a Phase 2 clinical study for ovarian cancer, peritoneal cancer and fallopian tube cancer.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
LEQEMBI is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble (protofibril*) and insoluble forms of Aβ. LEQEMBI is the first and only approved treatment shown to reduce the rate of disease progression and to slow cognitive and functional decline by selectively binding to and eliminating the most toxic Aβ aggregates (protofibrils) that contribute to neurotoxicity in AD. In Japan, an application for marketing approval was filed and was designated for priority review in January 2023. Japan is the second country to grant approval, following the traditional approval in the U.S. in July 2023.
LEQEMBI’s approval is based on Phase 3 data from Eisai’s large, global Clarity AD clinical trial, in which LEQEMBI met its primary endpoint and all key secondary endpoints with statistically significant results and confirmed the clinical benefit of LEQEMBI. The primary endpoint was the global cognitive and functional scale, Clinical Dementia Rating Sum of Boxes (CDR-SB). In the Clarity AD clinical trial, treatment with LEQEMBI reduced clinical decline on CDR-SB by 27% at 18 months compared to placebo. In addition, the secondary endpoint from the AD Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS MCI-ADL), which measures information provided by people caring for patients with AD, noted a statistically significant benefit of 37% compared to placebo. The ADCS MCI-ADL assesses the ability of patients to function independently, including being able to dress, feed themselves and participate in community activities. The most common adverse events (>10%) in the LEQEMBI group were infusion reactions, ARIA-H (combined cerebral microhemorrhages, cerebral macrohemorrhages, and superficial siderosis), ARIA-E (edema/effusion), headache, and fall. Full results of the Clarity AD study were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) 2022 conference and simultaneously published in the peer-reviewed medical journal The New England Journal of Medicine on November 29, 2022.
“Today LEQEMBI received approval, making it the first approved anti-amyloid Alzheimer’s disease treatment shown to reduce the rate of disease progression and to slow cognitive impairment in the early and mild dementia stages of the disease in Japan. We believe that we have turned a new page in the history of Alzheimer’s disease treatment. Alzheimer’s disease is a progressive and serious disease that not only causes significant impairment and burden for the people living with it and their care partners, but also has a tremendous impact on society as a whole,” said Haruo Naito, Chief Executive Officer at Eisai. “For around 40 years since we began research on dementia at our Tsukuba Research Laboratories, Eisai has interacted with people with dementia and their care partners, and made efforts to understand their sincere feelings. In response, we have been taking on the challenge to develop therapeutic agents that act on the underlying pathology of Alzheimer’s disease. We are committed to delivering LEQEMBI to the people with early Alzheimer’s disease who need it and their families as a new treatment that removes the cause of the disease. Through these efforts, we aim to create impact on issues surrounding dementia in Japanese society.”
“With this approval, alongside Eisai, we will be able to help address the devastating impact Alzheimer’s has on people living with the condition as well the emotional, social and financial burden it places on care partners,” said Christopher A. Viehbacher, President and Chief Executive Officer of Biogen. “This is a significant step in the work of Biogen and Eisai to usher in a new era of treatments for this disease which impacts millions. We look forward to working alongside Eisai to build on the approvals in the U.S. and now Japan to bring this option to patients and their families worldwide.”
Eisai will conduct a post-marketing special use results survey (all-case surveillance) in all patients who are administered LEQEMBI until data from a certain number of patients are accumulated after market launch, in accordance with an approval condition imposed by the Ministry of Health, Labour and Welfare. In addition, the appropriate use of LEQEMBI will be promoted in accordance with the package insert and training materials will be developed for healthcare professionals to assist the management and monitoring of amyloid-related imaging abnormalities (ARIA).
Eisai serves as the lead of LEQEMBI development and regulatory submissions globally with both Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority. In Japan, Eisai and Biogen Japan will co-promote LEQEMBI, with Eisai distributing the product as the Marketing Authorization Holder.
Media Contacts:
Eisai
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
Eisai Inc. (U.S.)
Libby Holman
+ 1-201-753-1945
Libby_Holman@eisai.com
Biogen Inc.
Jack Cox
+ 1 781-464-3260
public.affairs@biogen.com
Investor Contacts:?
Eisai Co., Ltd.
Investor Relations Department
TEL: +81 (0) 3-3817-5122
Biogen Inc.
Chuck Triano
+ 1-781-464-2442
IR@biogen.com
With the aim of relieving the anxieties of people with dementia and their families and addressing social issues, Eisai is not only creating therapeutic drugs, but also working to build a dementia ecosystem through the development of digital solutions and collaboration with other industries. Theoria technologies will serve as the core of a highly transparent and neutral dementia platform, and the foundation for the development of an ecosystem to empower the people with dementia, regardless of the type or stage of the disease, to “live their fullest lives”.
Theoria technologies will accelerate decision-making and strengthen the hiring and training of digital talent under an organizational structure optimized for digital business, and will utilize clinical study data that Eisai has accumulated over many years as well as cohort study data, Personal Health Records (PHR), and other data to develop various prediction algorithms, create digital solutions and provide data. Theoria technologies will commence business activities in April 2024, with the aim of providing services for a risk prediction algorithm for early detection of mild cognitive impairment (MCI) and dementia in FY2024. In addition, together with Eisai, Theoria technologies will develop and provide Sasaeru, an application that helps facilitate communication between people with dementia, doctors, and caregivers by recording Activities of Daily Living (ADL) of the people with dementia. Furthermore, Theoria technologies will promote the development of digital services by strengthening collaboration with other companies.
Eisai is aiming to create social impact by realizing a Dementia Inclusive Society where people with dementia and the people in the daily living domain can live their lives how they would like, through the development of an ecosystem.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
Joint Statement:
Today, the First Committee on New Drugs of the Pharmaceutical Affairs and Food Sanitation Council of the Ministry of Health, Labour and Welfare (MHLW) has recommended that lecanemab’s manufacturing and marketing authorization application for the treatment of early Alzheimer’s disease should be approved, which is a major step forward in the treatment of Alzheimer’s disease in Japan. We would like to express our deepest gratitude to all patients, caregivers, and healthcare professionals who participated in the lecanemab clinical trials. Once approved, we will do our best to appropriately deliver lecanemab, which was the first to demonstrate a clear reduction in the rate of Alzheimer’s disease progression and slowing of cognitive and functional decline, as a new treatment option for patients suffering from early Alzheimer’s disease in Japan.
]]>The FTSE4Good Index Series was developed by FTSE Russell to promote investment in companies that meet global environmental, social and governance (ESG) standards. Eisai received particularly high scores in “Corporate Governance”, “Anti-Corruption”, “Tax Transparency”, “Labor Standards” and “Customer Responsibility”, among others. As of the end of June 2023, 1,121 companies worldwide and 250 Japanese companies were included in the FTSE4Good Developed Index Series.
Currently, in addition to the MSCI ESG Leaders Indexes, another global ESG investment index, Eisai is also listed in the FTSE Blossom Japan Index, the FTSE Blossom Japan Sector Relative Index, the MSCI Japan ESG Select Leaders Index, the MSCI Japan Empowering Women Index (WIN) and the S&P/JPX Carbon Efficient Index, which are ESG investment indices for Japanese stocks adopted by the Government Pension Investment Fund (GPIF).
Eisai’s corporate concept is to give first thought to patients and people in the daily living domain, and to increase the benefits that health care provides to them, as well as address diverse healthcare needs worldwide. By strengthening its ESG initiatives and increasing non-financial value, Eisai is striving to sustainably enhance corporate value based on this concept.
For more information on our ESG initiatives, please visit https://www.eisai.com/sustainability/index.html.
Also, we post and share ESG-related information on Twitter, LinkedIn and Facebook.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
“At AAIC 2023 Eisai will present the latest data on lecanemab, an anti-Aβ protofibril antibody, that recently received traditional approval in the U.S. for patients with mild cognitive impairment (MCI) due to AD and mild AD. Leqembi was studied in a broad population, which included a mix of racial and ethnic groups and patients with common comorbid conditions and concomitant medications.” Additionally, Eisai will present important new data on E2814, an anti-MTBR tau antibody, which is currently in Phase II/III clinical trials with the Dominantly Inherited Alzheimer’s Network Trials Unit at Washington University St. Louis,” said Michael Irizarry, M.D., Deputy Chief Clinical Officer and Senior Vice President of Clinical Research, Alzheimer’s Disease and Brain Health, Eisai Inc. “As part of Eisai’s commitment to transparency and our human health care (hhc) and ecosystem mission, we will continue to present and publish data and information about our AD pipeline and research.”

Eisai serves as the lead of lecanemab development and regulatory submissions globally with both companies co-commercializing and co-promoting the product and Eisai having final decision-making authority.
This release discusses investigational uses of agents in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that such investigational agents will successfully complete clinical development or gain health authority approval.
MEDIA CONTACTS:
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
Eisai Inc. (U.S.)
Libby Holman
+ 1-201-753-1945
Libby_Holman@Eisai.com
Eisai Europe, Ltd.
(UK, Europe, Australia, New Zealand and Russia)
EMEA Communications Department
+44 (0) 786 601 1272
EMEA-comms@eisai.net
The Centers for Medicare & Medicaid Services (CMS) announced broader Medicare coverage of LEQEMBI
TOKYO and CAMBRIDGE, Mass., July 6, 2023 – Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) and Biogen Inc. (Nasdaq: BIIB, Corporate headquarters: Cambridge, Massachusetts, CEO: Christopher A. Viehbacher, “Biogen”) announced today that the U.S. Food and Drug Administration (FDA) has approved the supplemental Biologics License Application (sBLA) supporting the traditional approval of LEQEMBI? (lecanemab-irmb) 100 mg/mL injection for intravenous use, making LEQEMBI the first and only approved treatment shown to reduce the rate of disease progression and to slow cognitive and functional decline in adults with Alzheimer’s disease (AD). LEQEMBI demonstrated clinically meaningful slowing of cognitive and functional decline in a patient group generalizable to U.S. Medicare beneficiaries, which included a mix of racial and ethnic groups, patients with common comorbid conditions, concomitant medications and patients with mild cognitive impairment (MCI) due to AD or mild AD. Treatment with LEQEMBI should be initiated in patients with MCI or mild dementia stage of disease, (collectively referred to as early AD) the population in which treatment was initiated in clinical trials.
LEQEMBI’s traditional approval is based on Phase 3 data from Eisai’s large, global Clarity AD clinical trial, in which LEQEMBI met its primary endpoint and all key secondary endpoints with statistically significant results and confirmed the clinical benefit of LEQEMBI. The primary endpoint was the global cognitive and functional scale, Clinical Dementia Rating Sum of Boxes (CDR-SB). LEQEMBI treatment reduced clinical decline on CDR-SB by 27% at 18 months compared to placebo. Additionally, the secondary endpoint of AD Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS MCI-ADL), as measured by people caring for patients with AD, noted a statistically significant benefit of 37%. This measures the ability of patients to function independently, including being able to dress, feed themselves and participate in community activities. Full results of the Clarity AD study were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) 2022 conference and simultaneously published in the peer-reviewed medical journal The New England Journal of Medicine on November 29, 2022.
Importantly, following FDA’s traditional approval of LEQEMBI, CMS confirmed that broader coverage of LEQEMBI is now available and released more details on the registry, including the easy-to-use data submission process. The CMS-facilitated registry is now available for healthcare professionals to submit required patient data to CMS. Eisai is pleased that Medicare will cover this important therapy for appropriate patients. This will facilitate reimbursement for and access to LEQEMBI across a broad range of healthcare settings in the United States.
“Today, the FDA approved LEQEMBI under the traditional approval pathway, making LEQEMBI the first and only approved anti-amyloid Alzheimer’s disease treatment shown to reduce the rate of disease progression and to slow cognitive impairment in the early and mild dementia stages of the disease. As a research and development-focused company based on our hhc (human health care) concept, we are proud that the results of Eisai’s AD research over the past 40 years have been recognized and delivered to people living with this disease in the United States,” said Haruo Naito, Chief Executive Officer at Eisai. “Alzheimer’s disease is a progressive, fatal disease that greatly impacts not only the people living with it, but also their loved ones, care partners and society. We continue to work to create broad and simple access to LEQEMBI for patients and to support diagnosis and treatment at the early stage of the disease. Eisai will diligently work to educate physicians on the safe and appropriate use of LEQEMBI to maximize its benefit to people living with early AD and their families.”
“Today marks a breakthrough in the treatment of Alzheimer’s disease, and we are proud to be at the forefront of ushering in a new era of advances for a disease that was previously considered untreatable. We would like to express our sincere appreciation to those who have worked tirelessly to find a treatment for this unrelenting disease, without whom this progress would not be possible,” said Christopher A. Viehbacher, President and Chief Executive Officer of Biogen. “Our focus is now on the path forward, working alongside Eisai with the goal of making LEQEMBI accessible to eligible patients as soon as possible.”
LEQEMBI is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble (protofibril*) and insoluble forms of amyloid beta (Aβ). Critically, LEQEMBI targets and clears the most neurotoxic form of Aβ that continuously accumulates as well as removes the existing plaques to treat this progressive, chronic disease. In June 2023, the FDA’s Peripheral and Central Nervous System Drugs (PCNS) advisory committee voted unanimously that the data from Eisai’s Clarity AD clinical trial confirmed the clinical benefit of LEQEMBI for the treatment of AD. Committee members also confirmed the overall risk-benefit of LEQEMBI. On January 6, 2023, LEQEMBI was approved by the FDA under the accelerated approval pathway.
Eisai has developed and deployed Understanding ARIA , a multi-faceted educational initiative to further advance understanding in the AD healthcare community of the real-world management and monitoring of amyloid-related imaging abnormalities (ARIA). In collaboration with experts in the field of medical imaging as well as major professional societies, Understanding ARIA
, a multi-faceted educational initiative to further advance understanding in the AD healthcare community of the real-world management and monitoring of amyloid-related imaging abnormalities (ARIA). In collaboration with experts in the field of medical imaging as well as major professional societies, Understanding ARIA offers resources and programs that include peer-to-peer education, individual and group educational sessions and subject matter-expert evaluation of historical case studies.
offers resources and programs that include peer-to-peer education, individual and group educational sessions and subject matter-expert evaluation of historical case studies.
Eisai is committed to ensuring that appropriate patients have access to LEQEMBI and has established a Patient Assistance Program to provide LEQEMBI at no cost, for eligible uninsured and underinsured patients, including Medicare beneficiaries, who meet financial need and other program criteria. Additionally, Eisai offers patient support for improving access through LEQEMBI Patient Navigators, who will provide information about accessing LEQEMBI, help patients and their families understand their insurance coverage and options, and identify financial support programs for eligible patients. People in the U.S. can learn more about these services by visiting LEQEMBI.com, calling 1-833-4- LEQEMBI (1-833-453-7362), Monday-Friday, 8 a.m. to 8 p.m. Eastern Time or faxing an enrollment form to 1-833-770-7017.
Eisai serves as the lead of LEQEMBI development and regulatory submissions globally with both Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority.
Media Contacts:
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
Biogen Inc.
Jack Cox
+ 1 781-464-3260
public.affairs@biogen.com
Eisai Inc. (U.S.)
Libby Holman
+ 1-201-753-1945
Libby_Holman@eisai.com
Eisai Europe, Ltd.
(UK, Europe, Australia, New Zealand and Russia)
EMEA Communications Department
+44 (0) 786 601 1272
EMEA-comms@eisai.net
Investor Contacts:
Eisai Co., Ltd.
Investor Relations Department
TEL: +81 (0) 3-3817-5122
Biogen Inc.
Chuck Triano
+ 1-781-464-2442
IR@biogen.com
TOKYO, HATFIELD, SEATTLE, LONDON, and EDINBURGH, 29 June, 2023 – Eisai, Gates Ventures, Health Data Research UK (HDR UK), LifeArc and The University of Edinburgh announced today a new two-year collaborative research agreement. The collaboration, named NEURii is a unique pioneering partnership that creates a powerhouse collaboration of collective expertise in therapeutics, technology development and commercialization, health data management and advanced analytics/data science to predict, protect and promote brain health.
NEURii will focus its initial efforts to develop data and digital solutions to complement approved treatment options for patients and solve issues related to the prediction, prevention, management, and treatment of dementia related disorders.
This groundbreaking collaboration will use high-quality individual data, Artificial Intelligence (AI), and Machine Learning (ML) to deliver patient-focused digital health solutions by developing initial pilot projects originated in highly recognized UK academic centres. These projects have been selected on the basis of their potential to make a meaningful difference to patients’ lives while maintaining data security and public trust. By combining diverse digital biomarkers that can be acquired non-invasively in real world clinical and non-clinical settings (e.g., speech from conversation) with the high-quality and abundant medical data accumulated in the UK, and analyzing them with tailored AI algorithms, NEURii will create innovative digital solutions. These will be deployed in the detection, monitoring and treatment of dementia patients in order to improve their lives as well as minimizing the impact of the disease burden on their carers and families.
This initial two-year pilot establishes a first-in-class launch pad underpinned by an innovative business model and scalable prototype for translating scientific prototypes that will enhance and improve public health demonstrating real world impact. It is envisaged that NEURii partners will explore further opportunities to scale up the program developing digital health solutions worldwide.
Dr. Teiji Kimura, Ph.D., Academia and Industry Alliance Officer, Deep Human Biology Learning (DHBL) Office of Eisai, commented, “Dementia is one of the major social and medical issues in an aging society, and Eisai’s mission is to contribute to solving these issues. We aim to create new digital solutions that will contribute to solving the challenges of dementia by combining the UK’s leadership in this field with our experience and track record of continuously creating innovative treatments in the field of dementia whilst staying true to our human health care concept of giving first thought to patients and the people in the daily living domain.”
It is estimated that more than 55 million are currently living with dementia in the world, and nearly one million people in the UK, and this number is expected to grow rapidly.1,2 As well as having a significant impact on the lives of patients and those who care for them (52% of the UK public knows someone who has been diagnosed with a form of dementia), these conditions place significant pressure on health and social care systems. Providing data-driven solutions that complement existing treatments could help to improve earlier detection and diagnosis, evidence-based treatment decision-making, monitoring of disease progression and maintenance of quality of life.
“AI and other advanced technologies are beginning to play a powerful role in medical research,” said Dr. Niranjan Bose, Managing Director of Health & Life Sciences at Gates Ventures. “I’m excited about how the NEURii collaboration will apply these tools to diagnostics research and drug discovery, and contribute to breakthroughs that can improve life for millions of people suffering with dementia and dementia-related illnesses.”
“Identifying ways to prevent dementia and neurodegenerative disease is a key part of our multi-million-pound neurodegeneration program’ said Paul Wright, MND Translational Challenge Lead at LifeArc “‘This collaboration is one of many new innovative projects we are involved in to improve the diagnosis of dementia and a positive step towards predicting those who may develop the disease.”
The UK is a leader in digital technology investment and research across areas such as genomics, health data science, AI and ML, with rich and diverse health-related data. NEURii’s model will enable the identification of pioneering data and digital science, the mentoring of talented scientists and the translation of health prototypes into practical and accessible products. By bringing together the expertise and capabilities of the NEURii collaborators, it is hoped that the novel approach will provide an exciting launch-pad for new transformational digital products that can contribute to solving the ongoing challenges of dementia and neurodegenerative conditions.
Professor Andrew Morris, Director of HDR UK, said: “Almost one million people in the UK are living with dementia. This new public-private partnership aims to gain a deeper understanding of the disease through trustworthy use of large datasets of anonymised health data in secure environments. We will take forward a set of pilot projects and engage with the public. Our aim is to produce new data-driven products that will benefit patients and their families in detecting dementia, predicting its progress and better managing the disease.”
NEURii academic lead Professor Siddharthan Chandran, of the University of Edinburgh, said: “The University of Edinburgh is delighted to be part of this ambitious cross-sector digital partnership that has, as an explicit goal, the creation of low cost and globally scalable digital tools to predict and monitor dementia.”
Outputs from the NEURii collaboration will be shared quarterly internally and released externally when appropriate.
1? World Health Organization. Fact sheets, Dementia
https://www.who.int/news-room/fact-sheets/detail/dementia
2 Alzheimer’s Research UK. Dementia Statistics Hub. Number of people in the UK. 2022. Available at: https://dementiastatistics.org/about-dementia/prevalence-and-incidence/
MEDIA CONTACTS:
Eisai Co., Ltd.
Public Relations Department
+81-(0)3-3817-5120
Eisai Europe, Ltd.
Bily Kuo
Director, Communications EMEA
emea-comms@eisai.net
+44 (0) 7739 600 678
For queries relating to any of the signing partners: UoE, Gates Ventures, LifeArc and HDRUK
University of Edinburgh/Edinburgh Innovations
Megan Welford
Communications Manager
Megan.welford@ei.ed.ac.uk
+44 07721 120217